When it comes to choosing the right battery for your application, you likely have a list of conditions you need to fulfill. How much voltage is needed, what is the capacity requirement, cyclic or standby, etc.
Once you have the specifics narrowed down you may be wondering, “do I need a lithium battery or a traditional sealed lead acid battery?” Or, more importantly, “what is the difference between lithium and sealed lead acid?” There are several factors to consider before choosing a battery chemistry, as both have strengths and weaknesses.
For the purpose of this blog, lithium refers to Lithium Iron Phosphate (LiFePO4) batteries only, and SLA refers to lead acid/sealed lead acid batteries.
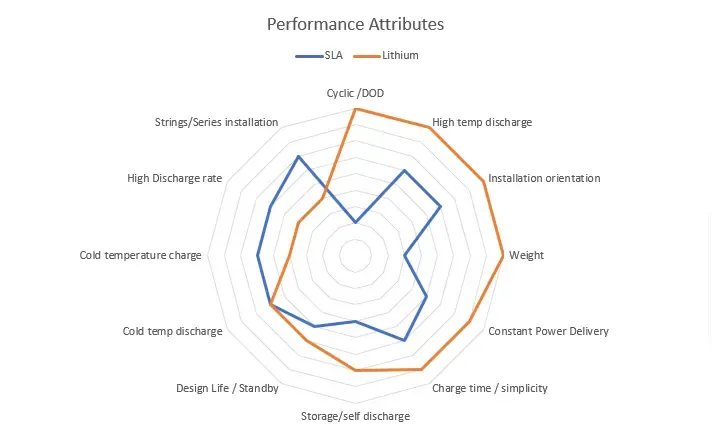
CYCLIC PERFORMANCE LITHIUM VS SLA
The most notable difference between lithium iron phosphate and lead acid is the fact that the lithium battery capacity is independent of the discharge rate. The figure below compares the actual capacity as a percentage of the rated capacity of the battery versus the discharge rate as expressed by C (C equals the discharge current divided by the capacity rating). With very high discharge rates, for instance .8C, the capacity of the lead acid battery is only 60% of the rated capacity. Find out more about C rates of batteries.
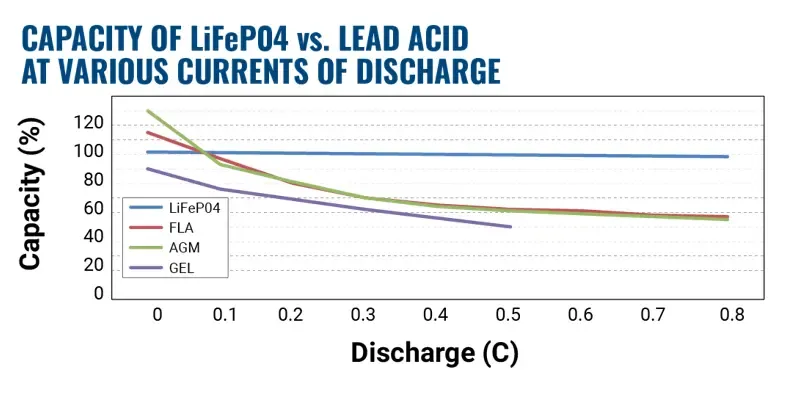
Therefore, in cyclic applications where the discharge rate is often greater than 0.1C, a lower rated lithium battery will often have a higher actual capacity than the comparable lead acid battery. This means that at the same capacity rating, the lithium will cost more, but you can use a lower capacity lithium for the same application at a lower price. The cost of ownership when you consider the cycle, further increases the value of the lithium battery when compared to a lead acid battery.
The second most notable difference between SLA and Lithium is the cyclic performance of lithium. Lithium has ten times the cycle life of SLA under most conditions. This brings the cost per cycle of lithium lower than SLA, meaning you will have to replace a lithium battery less often than SLA in a cyclic application.

CONSTANT POWER DELIVERY LITHIUM VS LEAD ACID
Lithium delivers the same amount of power throughout the entire discharge cycle, whereas an SLA’s power delivery starts out strong, but dissipates. The constant power advantage of lithium is shown in the graph below which shows voltage versus the state of charge.
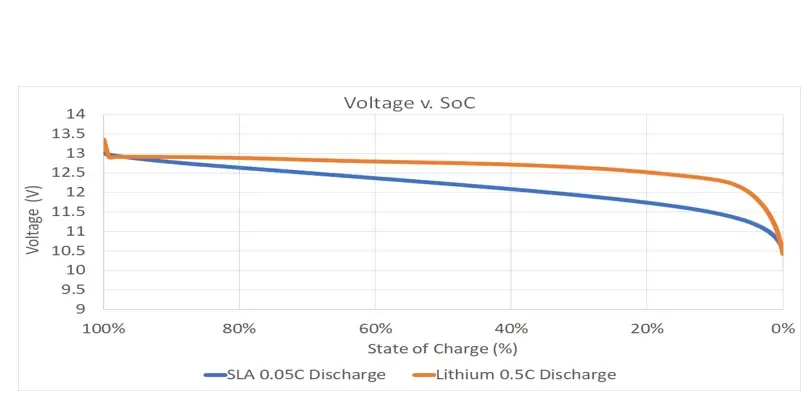
A lithium battery as shown in the orange has a constant voltage as it discharges throughout the entire discharge. Power is a function of voltage times current. The current demand will be constant and thus the power delivered, power times current, will be constant. So, let’s put this in a real-life example.
Have you ever turned on a
flashlight and noticed it’s dimmer than the last time you turned it on? This is
because the battery inside the flashlight is dying, but not yet completely dead.
It is giving off a little power, but not enough to fully illuminate the bulb.
If this were a lithium battery, the bulb would be just as bright from the beginning of its life to the end. Instead of waning, the bulb would just not turn on at all if the battery were dead.
CHARGING TIMES OF LITHIUM AND SLA
Charging SLA batteries is notoriously slow. In most cyclic applications, you need to have extra SLA batteries available so you can still use your application while the other battery is charging. In standby applications, an SLA battery must be kept on a float charge.
With lithium batteries, charging is four times faster than SLA. The faster charging means there is more time the battery is in use, and therefore requires less batteries. They also recover quickly after an event (like in a backup or standby application). As a bonus, there is no need to keep lithium on a float charge for storage. For more information on how to charge a lithium battery, please view our Lithium Charging Guide.
HIGH TEMPERATURE BATTERY PERFORMANCE
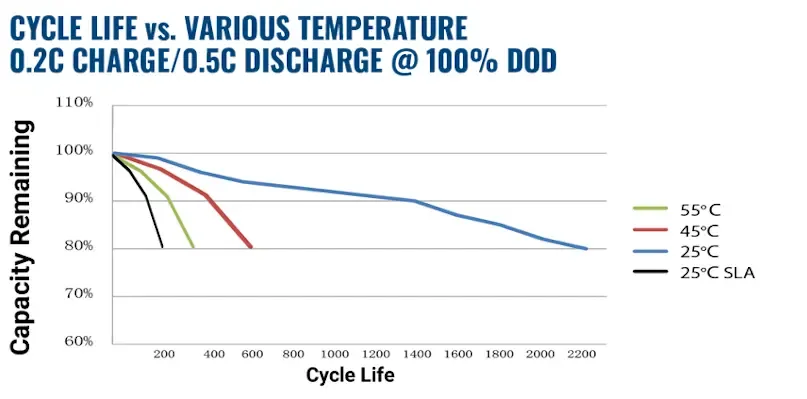
Lithium’s performance is far superior than SLA in high temperature applications. In fact, lithium at 55°C still has twice the cycle life as SLA does at room temperature. Lithium will outperform lead under most conditions but is especially strong at elevated temperatures.
COLD TEMPERATURE BATTERY PERFORMANCE
Cold temperatures can cause significant capacity reduction for all battery chemistries. Knowing this, there are two things to consider when evaluating a battery for cold temperature use: charging and discharging. A lithium battery will not accept a charge at a low temperature (below 32° F). However, an SLA can accept low current charges at a low temperature.
Conversely, a lithium battery has a higher discharge
capacity at cold temperatures than SLA. This means that lithium batteries do
not have to be over designed for cold temperatures, but charging could be a
limiting factor. At 0°F, lithium is discharged at 70% of its rated capacity,
but SLA is at 45%.
One thing to consider in cold temperature is the state of the lithium battery when you want to charge it. If the battery has just finished discharging, the battery will have generated enough heat to accept a charge. If the battery has had a chance to cool down, it may not accept a charge if the temperature is below 32°F.
BATTERY INSTALLATION
If you have ever tried to install a lead acid battery, you know how important it is to not install it in an invert position to prevent any potential issues with venting. While an SLA is designed to not leak, the vents allow for some residual release of the gasses.
In a lithium battery design, the cells are all individually sealed and cannot leak. This means there is no restriction in the installation orientation of a lithium battery. It can be installed on its side, upside down, or standing up with no issues.
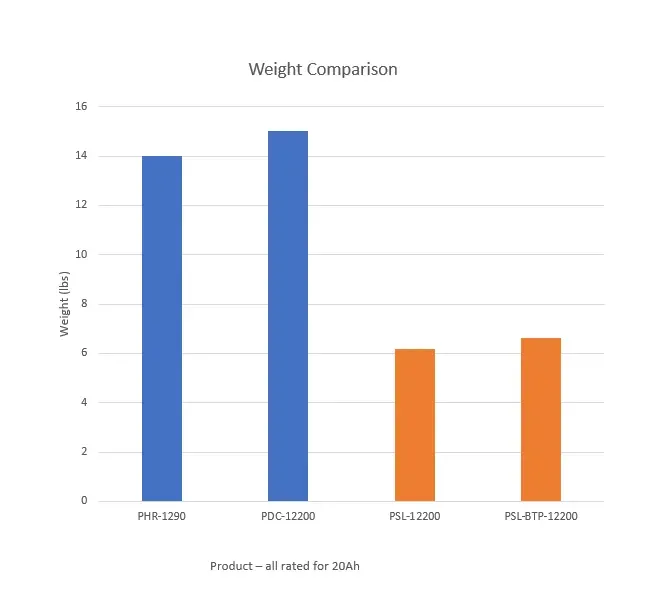
BATTERY WEIGHT COMPARISION
Lithium, on average, is 55% lighter than SLA. In cycling applications, this is especially important when the battery is being installed in a mobile application (batteries for motorcycles or scooters), or where weight may impact the performance (like in robotics). For standby use, weight is an important consideration in remote applications (solar fields) and where installation is difficult (up high in emergency lighting systems, for example).